How Many Grams Are in One Mole
This is why we state the atomic and. Check the chart for more details.

Mole Conversion Practice Worksheet The Best Worksheets Image Calculating Work Practices Worksheets Worksheets
The answer is 71080001.

. 6 What is the mass of 50 moles of Fe2O3. 4 How many moles of Fe2O3 are in 922 grams of Fe2O3. How to make molecular mass and mole concept What is the unit of mole.
This means that 1 mole of carbon dioxide has a mass of 4401 g. 1 grams KHP is equal to 0014068654838651 mole. You can always use our grams to.
Carbon dioxide contain one carbon and two oxygen atoms and. BYJUS Online learning Programs For K3 K10 K12 NEET. Oxygen has a molar mass of 160 g mol1 so 1 mole of oxygen atoms has a mass of 160 g.
Are mole and gram atoms the same. 3 How many grams are there in one mole of Fe2O3. 12 How many moles are in 68 grams of copper IT hydroxide CuOH2.
1 mole of Potassium Chlorate will produce 1 mole of Potassium Chloride. 2 How many grams of Fe2O3 are in 1 mol of Fe2O3. Calculate the mass in grams for the following.
The SI base unit for amount of substance is the mole. The atomic mass of an element is how many grams one mole weighs. Chemistry questions and answers.
5 How do you find the moles of Fe2O3. C 32 H 22 N 6 O 6 S 2 Na 2 69666 grams per mole ANSWER HERE 5. How many moles in an atom molar mass of one mole of alcl3 what is a solution.
30 moles 1 to grams 30 grams. 15 How many grams are in 23 x 10moles of calcium phosphate CaPO3. A mole can be defined as the molecular mass atomic mass and formula mass of a substance expressed in grams.
16 How many. 5 moles 1 to grams 5 grams. How many GRAMS of sulfur are present in 194 moles of sulfur hexafluoride SF6.
Text1 Gram Atomic Mass 1 Mole. For example carbon has an atomic weight of 12 amu so its molar mass is 12 grams per mole. Lithium for instance has an atomic mass of 6941 grams and this is equal to one mole of lithium.
11 What is the mole ratio of o2 G to CO2 g. 20 moles 1 to grams 20 grams. 14 How many moles are in 12 x 10 grams of ammonia NH3.
Basically the gram atom represents the atomic mass of the element in the grams. Do a quick conversion. And since there are.
10 moles 1 to grams 10 grams. We assume you are converting between grams KHP and mole. Consequently how many moles of KHP are in 0653 g.
75 moles 1 to grams. Molecular weight of KBr or grams This compound is also known as Potassium Bromide. You can view more details on each measurement unit.
It is also the average atomic weight of a substance. As you already know how the grams to moles conversion work find the number of moles. 8 How many grams are there in 5 moles of water.
1 mole is equal to 1 moles KBr or 1190023 grams. 1 mole is equal to 1 moles CuSO4 5H2O or 249685 grams. 12 How many.
10 What is the mole ratio of CO G to CO2 g. This expression means that 2 moles or 2 x 40 g 80 g of NaOH are dissolved in enough water to make one liter of solution. 11 How many grams are in 002 moles of beryllium iodide Bels.
The SI base unit for amount of substance is the mole. Mass of one Oxygen mole 16 g. 9 How do you calculate grams to moles.
There is a 11 mole ratio. Mass of one Carbon mole 12 g. 13 How many grams are in 33 moles of potassium sulfide K S.
You can view more details on each measurement unit. 1 mole is equivalent to 123895044 grams or 1 mole P4. N 5988 g 18015 gmol 3324 mol.
The molar mass is the amount of grams present in one mole of a substance. How many grams of O2 consists in 100 g of CO2. 8 How many grams are in 2 moles of CO.
5 How many moles is 40 grams of carbon. Atoms per mole of material we can calculate how many. 1 grams NaOH is equal to 0025001806380511 mole.
6022 1 0 23 6022 cdot 10 23 6022 1 0 23. It should be noted that rounding mistakes might occur therefore always double-check the findings. 7 How many moles are in 6 grams of carbon.
We assume you are converting between moles CuSO4 5H2O and gram. The reason is that one mole of the substance contains the amount of moles that are exactly in 12 grams of the carbon-12. You can view more details on each measurement unit.
Molecular weight of CuSO4 5H2O or grams The SI base unit for amount of substance is the mole. How many MOLES of fluorine are present in 299 grams of sulfur hexafluoride. 1 amu atom 1 g mol 1 frac text amu text atom 1 frac text g text mol 1 atom amu 1 mol g.
6 How many moles of carbon monoxide are present in molecules of this compound. 1 moles 1 to grams 1 grams. One mole of sodium is 2299 grams.
Molecular weight of KHP or mol The SI base unit for amount of substance is the mole. The formula for converting moles. A05 mol of calcium sulphate caSo4.
Refer to the textbook 8th ed Figure 27 p42 for explanation of moles Congo Red. This website will teach you how to convert moles of P4 to grams. 1 grams 1 1 mole using the molecular weight calculator and the molar mass of 1.
50 moles 1 to grams 50 grams. 1 mole of Na equals how many grams. In 1 kg-moles of any substance there exists exactly 103.
We assume you are converting between moles KBr and gram. One may also ask how many moles are in 80g of NaOH. 7 What is the mole mole ratio Fe2O3.
9 How many grams are in 240. 40 moles 1 to grams 40 grams. B 025 mol of carbon dioxide gas co2 cu40s32o16c12.
This means that the atomic mass or atomic weight 12 grams of carbon is equal to exactly 1 mole of carbon.
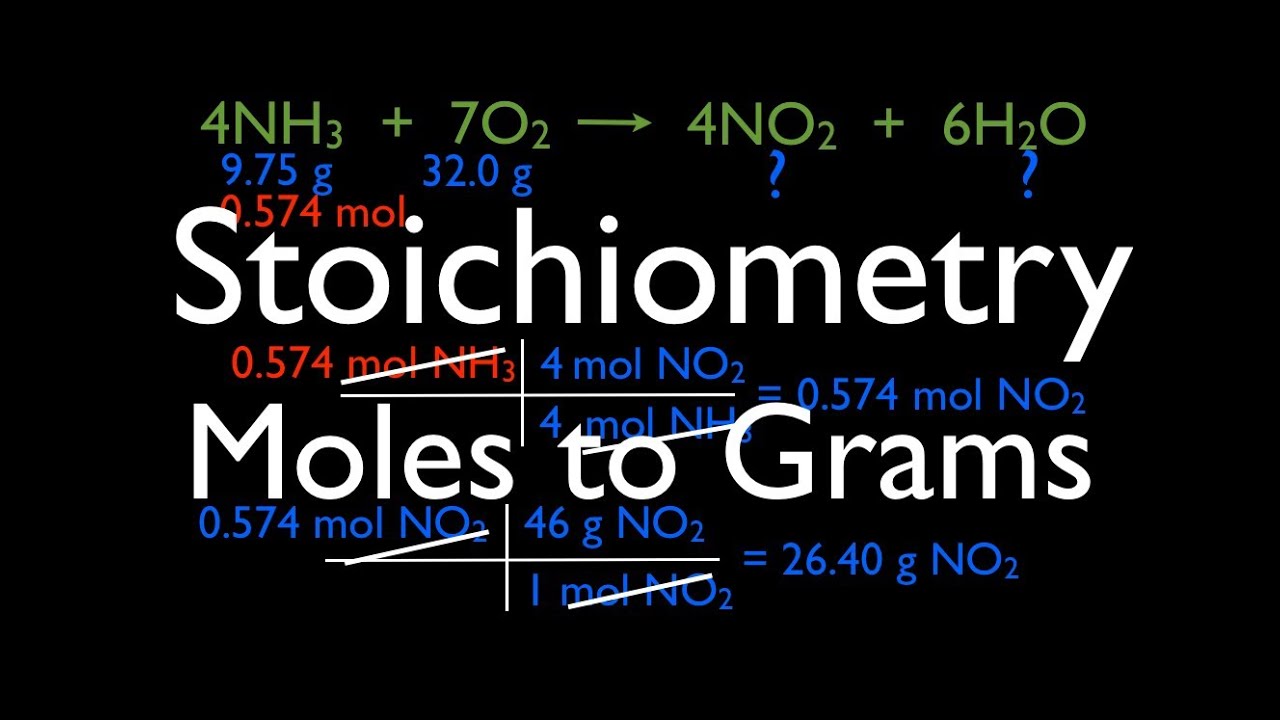
Stoichiometry Moles To Grams Youtube Chemistry Classroom Teaching Chemistry Science Chemistry

Mole Conversion Worksheet And Activity Iteachly Com Mole Conversion Worksheet Chemistry Lessons Teaching Chemistry

Increasing Access To Stoichiometry Through Differentiated In Class Practice Chemical Education Teaching Chemistry Chemistry Classroom Stoichiometry Chemistry

Mole Dominos Engaging Conversion Practice Chemistry Lessons Teaching Chemistry Chemistry Classroom

Stoichiometry And The Mole Test Unit Exam Exam Mole Concept The Unit

Xxssi Ibegetwccimage Jsp 973 1480 Teaching Chemistry Chemistry Lessons Chemistry Classroom

Molar Mass Is The Weight Of One Mole Of Any Chemical Compounds How Do You Calculate Molar Mass Of Element Hydrogen Mol Molar Mass Molars Teaching Chemistry

Avogadro Constant Is The Number 6 02x10 23 Get That In Your Head Ha Ha Chemistry Classroom Chemistry Chemistry Education

It S Mole Day Teaching Chemistry Chemistry Classroom Chemistry

Molar Mass Molar Mass Chemistry Lessons Science Chemistry

Avogadro S Constant Surfguppy Chemistry Made Easy Visual Learning Chemistry Lessons Chemistry Classroom Teaching Chemistry

What Does A Mole Mean Teaching Chemistry Chemistry Classroom Chemistry Activities

How Much Is A Mole Of Water Mass And Volume Mole Mole Day Chemistry Notes

7 12 Free Mole Conversion Reference Chart Trying To Explain When To Use Avogadro S Number Or Mo Writing Prompts For Kids Chemistry Lessons Teaching Chemistry

Mole Easy Science Chemistry Basics Mole Day Chemistry

Avogadros Number Easy Science Chemistry 10 Science Chemistry Easy Science

Moles 9 Moles Diphosphorus Pentoxide To Grams Diphosphorus Pentoxide Chemistry Help Chemistry Videos Tutorial

Moles Molecules Atoms Conversion Part 1 2 Mole Conversion Teacher Material Molecules

Converting Between Grams And Moles Part 2 Youtube Molar Mass Grams Physical Science
Comments
Post a Comment